Thursday, April 19, 2018
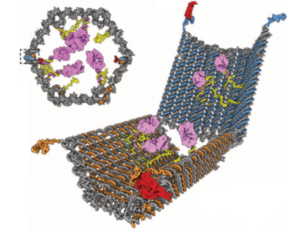
Viruses Modulate the Function and Evolution of All Living Things
By
CNu
at
April 19, 2018
0
comments
![]()
Labels: as above-so below , Exponential Upside , gain of function , Genetic Omni Determinism GOD , microbiome , microcosmos , What IT DO Shawty...
Saturday, April 15, 2017
2017 Website@Most Important Lab at Harvard and Arguably the World?!?!
| Harvard Molecular Technologies | Contact | Calendar | Courses | G.Church | Lab photos & List | News | Publications |
|
|---|
Journals: MIT, Harvard, Science , Pubmed History of this web page.
-->
By
CNu
at
April 15, 2017
0
comments
![]()
Labels: Ass Clownery , comedy gold , FAIL
Sunday, November 29, 2015
do you ever wonder what your anthropocene antics look like from the bacterial apex?
By
CNu
at
November 29, 2015
0
comments
![]()
Labels: as above-so below , microcosmos , paradigm , play-at-your-level
Thursday, October 08, 2015
N-1 a literal diseased state?
The modern anthropological view on religion is that it is a cultural meme that replicates through social communication [44]. While the meme itself may influence behavior, religious icons are known to be vectors of infectious diseases [45]. Most major religions have rituals that are likely to promote the transmission of infections. This includes circumcision [46], Christian common communion chalice [46], the Hindu ‘side-roll’[46] and Islamic ritual ablution [46] as well as the Hajj congregation in Mecca [47]. For example, the latter is specifically associated with outbreaks of meningococcal disease [48].
Thus it is possible that various religious practices could represent biomemes: manifestations of a symbiosis between informational memes [54] and biological organisms. This concept is somewhat similar to the fictional midichlorians of the Jedi Order from the popular series “Star Wars”[55].
Two particular parts of the human body seem to be most promising for the search of behavior-altering parasites. First of all, the human gut microbiome may be of interest in light of the microbiome-gut-brain axis concept. Another promising area to search for behavioraltering parasites is the human brain. Several organisms that can bypass the mammalian blood–brain barrier and produce a latent infection without obvious symptoms are currently known. In mice with latent toxoplasmosis, Toxoplasma gondii cysts can be found in various regions of the brain, especially in the olfactory bulb, the entorhinal, somatosensory, motor and orbital, frontal association and visual cortices, the hippocampus and the amygdala [56]. In humans the brain also appears to be an important site for Toxoplasma gondii cyst formation and the parasite is capable of infecting a variety of brain cells, including astrocytes and neurons [57-59].
By
CNu
at
October 08, 2015
0
comments
![]()
Labels: microbiome , microcosmos , Tard Bidnis , What IT DO Shawty...
the business model consists in fleecing credulous geeks with more money than sense...,
By
CNu
at
October 08, 2015
0
comments
![]()
Labels: Ass Clownery
Saturday, July 11, 2015
transhumans about the bidnis of enginnering biomes, as well...,
By
CNu
at
July 11, 2015
13
comments
![]()
Labels: microcosmos , symbiosis , synthesis , tactical evolution
Friday, September 05, 2014
the body's ecosystem
By
CNu
at
September 05, 2014
0
comments
![]()
Labels: microcosmos , symbiosis , What IT DO Shawty...
Wednesday, August 13, 2014
microbial colonization...,
By
CNu
at
August 13, 2014
0
comments
![]()
Labels: as above-so below , co-evolution , symbiosis , What IT DO Shawty...
Tuesday, August 12, 2014
voices from within: gut microbes and the central nervous system
By
CNu
at
August 12, 2014
1 comments
![]()
Labels: as above-so below , essence , microcosmos
missing microbes: conspicuously obvious once the man points it out...,
By
CNu
at
August 12, 2014
0
comments
![]()
Labels: as above-so below , common sense , microcosmos , symbiosis
the quantified microbiome visualization looks strangely like an appflow visualization...,
By
CNu
at
August 12, 2014
2
comments
![]()
Labels: microcosmos , symbiosis , tactical evolution
Saturday, March 01, 2014
biota, diet, brains, power...,
By
CNu
at
March 01, 2014
0
comments
![]()
Labels: food-powered , Genetic Omni Determinism GOD , microcosmos
Saturday, August 24, 2013
breath straight kicking like cancer....,
By
CNu
at
August 24, 2013
0
comments
![]()
Labels: microcosmos , shameless , subliminal
Sunday, April 07, 2013
human breath analysis and individual metabolic phenotypes
By
CNu
at
April 07, 2013
23
comments
![]()
Labels: microcosmos , symbiosis , What IT DO Shawty...
Friday, June 22, 2012
medical ecology
“I would like to lose the language of warfare,” said Julie Segre, a senior investigator at the National Human Genome Research Institute. “It does a disservice to all the bacteria that have co-evolved with us and are maintaining the health of our bodies.”
This new approach to health is known as medical ecology. Rather than conducting indiscriminate slaughter, Dr. Segre and like-minded scientists want to be microbial wildlife managers.
No one wants to abandon antibiotics outright. But by nurturing the invisible ecosystem in and on our bodies, doctors may be able to find other ways to fight infectious diseases, and with less harmful side effects. Tending the microbiome may also help in the treatment of disorders that may not seem to have anything to do with bacteria, including obesity and diabetes.
“I cannot wait for this to become a big area of science,” said Michael A. Fischbach, a microbiologist at the University of California, San Francisco, and an author of a medical ecology manifesto published this month in the journal Science Translational Medicine.
Judging from a flood of recent findings about our inner ecosystem, that appears to be happening. Last week, Dr. Segre and about 200 other scientists published the most ambitious survey of the human microbiome yet. Known as the Human Microbiome Project, it is based on examinations of 242 healthy people tracked over two years. The scientists sequenced the genetic material of bacteria recovered from 15 or more sites on their subjects’ bodies, recovering more than five million genes.
The project and other studies like it are revealing some of the ways in which our invisible residents shape our lives, from birth to death.
A number of recent reports shed light on how mothers promote the health of their children by shaping their microbiomes. In a study published last week in the journal PLoS One, Dr. Kjersti Aagaard-Tillery, an obstetrician at Baylor College of Medicine, and her colleagues described the vaginal microbiome in pregnant women. Before she started the study, Dr. Aagaard-Tillery expected this microbiome to be no different from that of women who weren’t pregnant.
“In fact, what we found is the exact opposite,” she said.
By
CNu
at
June 22, 2012
5
comments
![]()
Labels: microcosmos , What IT DO Shawty...
Monday, June 18, 2012
oops, they forgot the breastesses...,
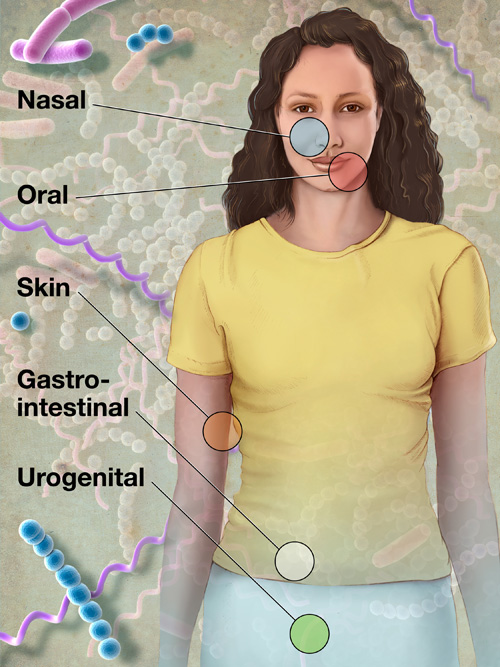 kurzweilai | Some 200 members of the Human Microbiome Project (HMP) Consortium from nearly 80 universities and scientific institutions, organized by the National Institutes of Health, have mapped the normal microbial makeup of healthy humans, producing numerous insights and even a few surprises.
kurzweilai | Some 200 members of the Human Microbiome Project (HMP) Consortium from nearly 80 universities and scientific institutions, organized by the National Institutes of Health, have mapped the normal microbial makeup of healthy humans, producing numerous insights and even a few surprises.The report on on their five years of research was published Thusday June 14, 2012, in a series of coordinated scientific reports in Nature the PLoS.
Researchers found, for example, that nearly everyone routinely carries pathogens, microorganisms known to cause illnesses.
In healthy individuals, however, pathogens cause no disease; they simply coexist with their host and the rest of the human microbiome, the collection of all microorganisms living in the human body.
Researchers must now figure out why some pathogens turn deadly and under what conditions, likely revising current concepts of how microorganisms cause disease.
“Like 15th century explorers describing the outline of a new continent, HMP researchers employed a new technological strategy to define, for the first time, the normal microbial makeup of the human body,” said NIH Director Francis S. Collins, M.D., Ph.D.
“HMP created a remarkable reference database by using genome sequencing techniques to detect microbes in healthy volunteers. This lays the foundation for accelerating infectious disease research previously impossible without this community resource.”
To define the normal human microbiome, HMP researchers sampled 242 healthy U.S. volunteers (129 male, 113 female), collecting tissues from 15 body sites in men and 18 body sites in women.
Researchers collected up to three samples from each volunteer at sites such as the mouth, nose, skin (two behind each ear and each inner elbow), and lower intestine (stool), and three vaginal sites in women; each body site can be inhabited by organisms as different as those in the Amazon Rainforest and the Sahara Desert.
Historically, doctors studied microorganisms in their patients by isolating pathogens and growing them in culture. This painstaking process typically identifies only a few microbial species, as they are hard to grow in the laboratory. In HMP, researchers purified all human and microbial DNA in each of more than 5,000 samples and analyzed them with DNA sequencing machines.
By
CNu
at
June 18, 2012
0
comments
![]()
Labels: microcosmos , What IT DO Shawty...
the wonder of breasts
 Guardian | We love breasts, yet can't quite take them seriously. Breasts embarrass us. They're unpredictable. They're goofy. They can turn babies and grown men into lunkheads.
Guardian | We love breasts, yet can't quite take them seriously. Breasts embarrass us. They're unpredictable. They're goofy. They can turn babies and grown men into lunkheads.They appear out of nowhere in puberty, they get bigger in pregnancy, they're capable of producing prodigious amounts of milk, and sometimes they get sick. But for such an enormously popular feature of the human race, it's remarkable how little we know about their basic biology.
The urgency to know and understand breasts has never been greater. Modern life has helped many of us live longer and more comfortably. It has also, however, taken a strange toll on our breasts. For one thing, they are bigger than ever. We are sprouting them at younger ages. We are filling them with saline and silicone and transplanted stem cells to change their shape. This year marks the 50th anniversary of the first silicone implant surgery in Houston, Texas.
More tumours form in the breast than in any other organ, making breast cancer the most common malignancy in women worldwide. Its incidence has almost doubled since the 1940s and is still rising.
But breasts are often overlooked, at least for non-cancer scientific research. The Human Microbiome Project, for example, is decoding the microbial genes of every major human gland, liquid and orifice, from the ears to the genitals. It neglected to include breast milk.
I wanted to know more, so I went to the 15th meeting of the International Society for Research in Human Milk and Lactation in Lima. Many attendees were molecular biologists, biochemists or geneticists who are deconstructing milk bit by bit. Until recently, it was thought breast milk had around 200 components. These could be divided into the major ingredients of fats, sugars, proteins and enzymes. But new technologies have allowed researchers to look deeper into each of these categories and discover new ones.
Scientists used to think breast milk was sterile, like urine. But it's more like cultured yoghurt, with lots of live bacteria doing who knows what. These organisms evolved for a reason, and somehow they're helping us out. One leading theory is they act as a vaccine, inoculating the infant gut. A milk sample has anywhere from one to 600 species of bacteria. Most are new to science.
Then there are the sugars. There's a class of them called oligosaccharides, which are long chains of complex sugars. Scientists have identified 140 of them so far, and estimate there are about 200. The human body is full of oligosaccharides, which ride on our cells attached to proteins and lipids. But a mother's mammary gland cooks up a unique batch of "free" or unattached ones and deposits them in milk. These are found nowhere else in nature, and not every mother produces the same ones, since they vary by blood type. Even though they're sugars, the oligosaccharides are, weirdly, not digestible by infants. Yet they are a main ingredient, present in milk in the same percentage as the proteins, and in higher amounts than the fats. So what are they doing there?
They don't feed us, but they do feed many types of beneficial bacteria that make a home in our guts and help us fight infections. In addition to recruiting the good bugs, these sugars prevent the bad bugs from hanging around. "The benefits of human milk are still underestimated," said Lars Bode, an immunobiologist at the University of California, San Diego. "We're still discovering functional components of breast milk."
By
CNu
at
June 18, 2012
1 comments
![]()
Labels: microcosmos , What IT DO Shawty...
Friday, May 18, 2012
what bugs are in your gut?
“It’s a humungous paper, with multiple key findings,” said food scientist David Mills of the University of California, Davis. “An impressive and complex piece of work,” agreed molecular biologist Jeremy Nicholson of Imperial College, London. Neither researcher participated in the study.
The scale and complexity stem from the research team’s aim of answering a multifaceted question—“What is the degree to which these microbial communities… vary within a person, as a function of postnatal development, physiological status, cultural tradition, and where a person lives,” said geneticist Jeffrey Gordon of the Washington University in St Louis, who led the study.
To this end, the researchers collected samples of feces from villagers in rural Malawi, Amerindians in Amazonian Venezuela, and metropolis-dwelling Americans. They then performed high-throughput sequencing on DNA taken from the samples to determine both the species and strains of microbes present and which microbial genes were most abundant.
The team found a common pattern for how the microbiomes of babies develop in the three countries. “It takes 6 to 9 months to get the first 6 or 700 bugs and then another couple of years to get the adult set,” explained Nicholson. “[Gordon] finds there is the same sort of developmental time span between countries,” he said, “but that the resulting microbiomes are nonetheless distinct between, let’s call it, a third-world population and a westernized population.”
One of the most striking differences was the degree of microbial diversity, with both the Amerindians and Malawians having far greater diversity than the Americans. “But, ironically, [Americans] might have more diversity in terms of the food eaten,” said Mills, which might have been expected to correlate with microbial diversity. Gordon suggested the Westerners’ lack of diversity could result from “our lifestyle, our degree of hygiene, [and] our use of antibiotics,” though further research is needed to test these possibilities.
Despite these differences between the gut microbiomes of the three cultures, there were also striking similarities, said Gordon. For example, “across all three populations, we see this age-dependent change in vitamin biosynthesis,” he said. In infants, gut bacteria tend to carry more copies of genes involved in folate biosynthesis, while the guts of older individuals harbor microbes carrying more genes for folate metabolism. Conversely, genes involved in vitamin B-12 synthesis became more prevalent in the gut microbiome with age.
“What’s really fascinating about those results,” said Mills, “is that it is reflecting what the host needs.”
By
CNu
at
May 18, 2012
0
comments
![]()
Labels: microcosmos , What IT DO Shawty...
Thursday, April 21, 2011
bacteria divide people into types
 NYTimes | In the early 1900s, scientists discovered that each person belonged to one of four blood types. Now they have discovered a new way to classify humanity: by bacteria. Each human being is host to thousands of different species of microbes. Yet a group of scientists now report just three distinct ecosystems in the guts of people they have studied.
NYTimes | In the early 1900s, scientists discovered that each person belonged to one of four blood types. Now they have discovered a new way to classify humanity: by bacteria. Each human being is host to thousands of different species of microbes. Yet a group of scientists now report just three distinct ecosystems in the guts of people they have studied.Blood type, meet bug type.
“It’s an important advance,” said Rob Knight, a biologist at the University of Colorado, who was not involved in the research. “It’s the first indication that human gut ecosystems may fall into distinct types.”
The researchers, led by Peer Bork of the European Molecular Biology Laboratory in Heidelberg, Germany, found no link between what they called enterotypes and the ethnic background of the European, American and Japanese subjects they studied.
Nor could they find a connection to sex, weight, health or age. They are now exploring other explanations. One possibility is that the guts, or intestines, of infants are randomly colonized by different pioneering species of microbes.
The microbes alter the gut so that only certain species can follow them.
Whatever the cause of the different enterotypes, they may end up having discrete effects on people’s health. Gut microbes aid in food digestion and synthesize vitamins, using enzymes our own cells cannot make.
Dr. Bork and his colleagues have found that each of the types makes a unique balance of these enzymes. Enterotype 1 produces more enzymes for making vitamin B7 (also known as biotin), for example, and Enterotype 2 more enzymes for vitamin B1 (thiamine).
The discovery of the blood types A, B, AB and O had a major effect on how doctors practice medicine. They could limit the chances that a patient’s body would reject a blood transfusion by making sure the donated blood was of a matching type. The discovery of enterotypes could someday lead to medical applications of its own, but they would be far down the road.
“Some things are pretty obvious already,” Dr. Bork said. Doctors might be able to tailor diets or drug prescriptions to suit people’s enterotypes, for example.
Or, he speculated, doctors might be able to use enterotypes to find alternatives to antibiotics, which are becoming increasingly ineffective. Instead of trying to wipe out disease-causing bacteria that have disrupted the ecological balance of the gut, they could try to provide reinforcements for the good bacteria. “You’d try to restore the type you had before,” he said.
Dr. Bork notes that more testing is necessary. Researchers will need to search for enterotypes in people from African, Chinese and other ethnic origins. He also notes that so far, all the subjects come from industrial nations, and thus eat similar foods. “This is a shortcoming,” he said. “We don’t have remote villages.”
The discovery of enterotypes follows on years of work mapping the diversity of microbes in the human body — the human microbiome, as it is known. The difficulty of the task has been staggering. Each person shelters about 100 trillion microbes.
(For comparison, the human body is made up of only around 10 trillion cells.) But scientists cannot rear a vast majority of these bacteria in their labs to identify them and learn their characteristics.
By
CNu
at
April 21, 2011
2
comments
![]()
Labels: microcosmos , What IT DO Shawty...
Wednesday, February 02, 2011
gut microbes influence behavior
 TheScientist | Gut microbes acquired early in life can impact brain development in mice and subsequent behavior, such as decreasing physical activity and increasing anxiety, according to a study published this week in the Proceedings of the National Academy of Sciences.
TheScientist | Gut microbes acquired early in life can impact brain development in mice and subsequent behavior, such as decreasing physical activity and increasing anxiety, according to a study published this week in the Proceedings of the National Academy of Sciences."This paper opens the door to new studies in at least two directions," Yale University microbiologist Andrew Goodman, who was not involved in the research, told The Scientist in an email. "First, determining how differences between complete host-associated microbial communities lead to differences in behavior, and second, exploring the contributions of microbes during specific developmental periods in the host."
Gut microbiota often colonize their hosts early in life, either during pregnancy or following birth, and play an integral role in the health of developing organisms. Previous research has shown that the bacteria affect the development of liver function, the protection epithelial cells afford underlying digestive tissue, gut regulation and the growth of new capillary blood vessels. But this is the first time gut flora have been linked to brain development and behavior.
Harmful microbial infections, on the other hand, have been linked to neurodevelopmental disorders, including autism and schizophrenia. And rodents infected by microbial pathogens before and after birth demonstrated behavioral abnormalities, such as anxiety-like behavior and impaired cognitive function, leading Rochellys Diaz Heijtz, a neurobiologist at the Karolinska Institute in Sweden, and her colleagues to wonder if the gut's normal microbial residents may similarly influence brain development.
The researchers tested exploratory activity in germ-free mice and mice with normal gut microbiota by tracking their movements across open space. They also tested anxiety of the two groups in two classic rodent behavioral tests -- the light-dark box and the elevated maze. Spending more time in lit areas and along unwalled, elevated maze portions equated to less anxiety.
Germ-free mice appeared to be more exploratory than mice with normal microbiota, venturing farther and to more areas of the space provided. Germ-free mice also spent more time in the light and engaged in riskier behavior in the maze, indicating they suffered from less anxiety than their microbe-filled counterparts.
The team then infected germ-free mice with normal gut microbiota when they were born to test whether the gut flora could alter the mice's activity and anxiety levels. Sure enough, the newly infected mice spent less time exploring and engaging in risky behavior, like the normal mice in the initial experiments. The results further supported the argument that the microorganisms can affect brain and behavior when introduced early enough in development.
"These microorganisms communicate in a systemic fashion to the developmental programming of a new individual and can influence fundamental aspects of behavior," said Diaz Heijtz. "We should start to consider the possibility that the microbiome and/or its composition may contribute to psychiatric problems."
By
CNu
at
February 02, 2011
1 comments
![]()
Labels: microcosmos , What IT DO Shawty...
Politicians Owned By The Tiny Minority Pass Bill To Protect Zionism
AP | The House passed legislation Wednesday that would establish a broader definition of antisemitism for the Department of Education t...

-
theatlantic | The Ku Klux Klan, Ronald Reagan, and, for most of its history, the NRA all worked to control guns. The Founding Fathers...
-
Video - John Marco Allegro in an interview with Van Kooten & De Bie. TSMATC | Describing the growth of the mushroom ( boletos), P...
-
Farmer Scrub | We've just completed one full year of weighing and recording everything we harvest from the yard. I've uploaded a s...